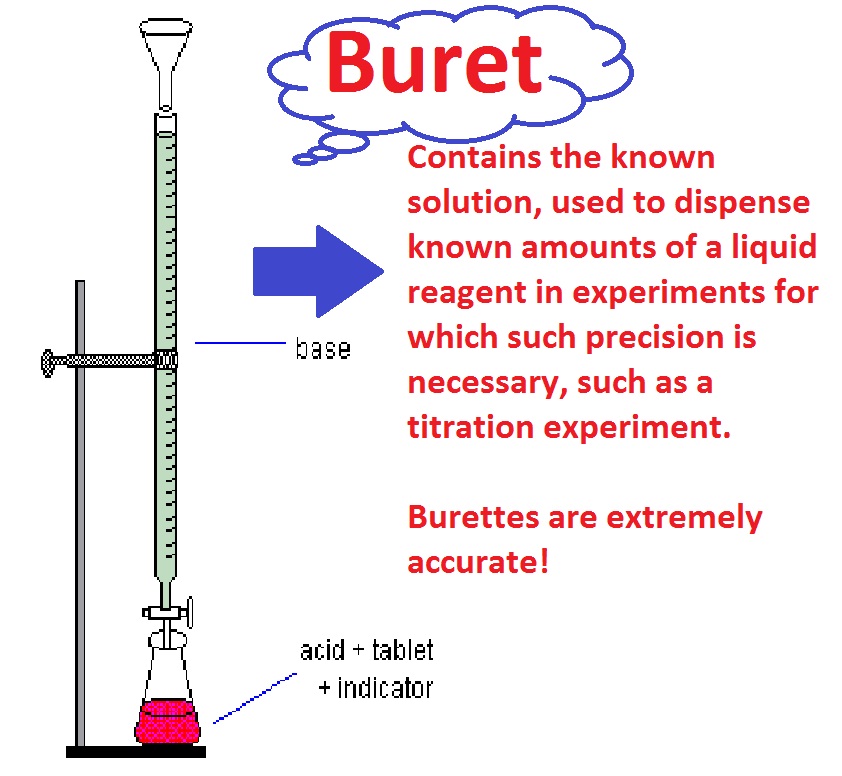
Titration Ka Meaning . titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. The chemical of unknown concentration is called the analyte or titrand. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. Determine the reacting volumes of solutions of acid and alkali by. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a method used to prepare salts if the reactants are soluble. titration is also known as titrimetry or volumetric analysis.
from cehpcvvh.blob.core.windows.net
titration is also known as titrimetry or volumetric analysis. The chemical of unknown concentration is called the analyte or titrand. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. Determine the reacting volumes of solutions of acid and alkali by. Titration is a method used to prepare salts if the reactants are soluble.
Titration Meaning In Chemistry at Donna Lewis blog
Titration Ka Meaning Titration is a method used to prepare salts if the reactants are soluble. Titration is a method used to prepare salts if the reactants are soluble. titration is also known as titrimetry or volumetric analysis. The chemical of unknown concentration is called the analyte or titrand. Determine the reacting volumes of solutions of acid and alkali by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Ka Meaning Determine the reacting volumes of solutions of acid and alkali by. The chemical of unknown concentration is called the analyte or titrand. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration is a method used to prepare salts if the reactants are soluble. a titration is a laboratory. Titration Ka Meaning.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Ka Meaning titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is also known as titrimetry or volumetric analysis. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. Titration is a method used. Titration Ka Meaning.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Ka Meaning titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is also known as titrimetry or volumetric analysis. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. The chemical of unknown concentration. Titration Ka Meaning.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Ka Meaning titration is also known as titrimetry or volumetric analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Determine the reacting volumes of solutions of acid and alkali by. The chemical of unknown concentration is called the analyte or titrand. Titration is a method used to prepare salts if. Titration Ka Meaning.
From www.youtube.com
AP Chemistry Ka Determination from Titration curves YouTube Titration Ka Meaning a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a method used to prepare salts if the reactants are soluble. The chemical of unknown concentration is called the analyte or titrand. titration is also known as titrimetry or volumetric analysis. titration, process of chemical. Titration Ka Meaning.
From learningschoolregibanb.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration Ka Meaning titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. The chemical of unknown concentration is called the analyte or titrand. Titration is a method used to prepare salts if the reactants are soluble. titration is also known as titrimetry or volumetric analysis.. Titration Ka Meaning.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog Titration Ka Meaning The chemical of unknown concentration is called the analyte or titrand. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a method used to prepare salts if the reactants are soluble. titration is also known as titrimetry or volumetric analysis. titration is a quantitative. Titration Ka Meaning.
From school.careers360.com
types of titration Overview, Structure, Properties & Uses Titration Ka Meaning titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. Determine the reacting volumes of solutions of acid and alkali by. titration is also. Titration Ka Meaning.
From www.studypool.com
SOLUTION Titrations titration meaning Studypool Titration Ka Meaning Determine the reacting volumes of solutions of acid and alkali by. The chemical of unknown concentration is called the analyte or titrand. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. titration is also known as titrimetry or volumetric analysis. Titration is. Titration Ka Meaning.
From www.slideserve.com
PPT Acid / Base Titration K a (HAc) PowerPoint Presentation, free Titration Ka Meaning The chemical of unknown concentration is called the analyte or titrand. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration is a method used to prepare salts if. Titration Ka Meaning.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Ka Meaning titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. Titration is a method used to prepare salts if the reactants are soluble. The chemical. Titration Ka Meaning.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Titration Ka Meaning titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Determine the reacting volumes of solutions of acid and alkali by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. a titration is. Titration Ka Meaning.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Ka Meaning Determine the reacting volumes of solutions of acid and alkali by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. The chemical of unknown concentration is called the analyte or titrand. Titration is a method used to prepare salts if the reactants are. Titration Ka Meaning.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Ka Meaning a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a method used to prepare salts if the reactants are soluble. titration is also known as titrimetry or volumetric analysis. Determine the reacting volumes of solutions of acid and alkali by. titration is a quantitative. Titration Ka Meaning.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Ka Meaning a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration Ka Meaning.
From learningschoolregibanb.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Ka Meaning The chemical of unknown concentration is called the analyte or titrand. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is also known as titrimetry or volumetric analysis. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution. Titration Ka Meaning.
From cekuuhzm.blob.core.windows.net
What Is The Principle Of Kf Titration at Sarah Patton blog Titration Ka Meaning The chemical of unknown concentration is called the analyte or titrand. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration is a method. Titration Ka Meaning.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Ka Meaning Titration is a method used to prepare salts if the reactants are soluble. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Determine the reacting volumes of solutions of acid and alkali by. titration, process of chemical analysis in which the quantity of some constituent of a. Titration Ka Meaning.